Case scenario

Helping consumers and health professionals make safe and wise decisions about medicines and diagnostics. Funded by the Australian Government through the Quality Use of Diagnostics, Therapeutics and Pathology Program.
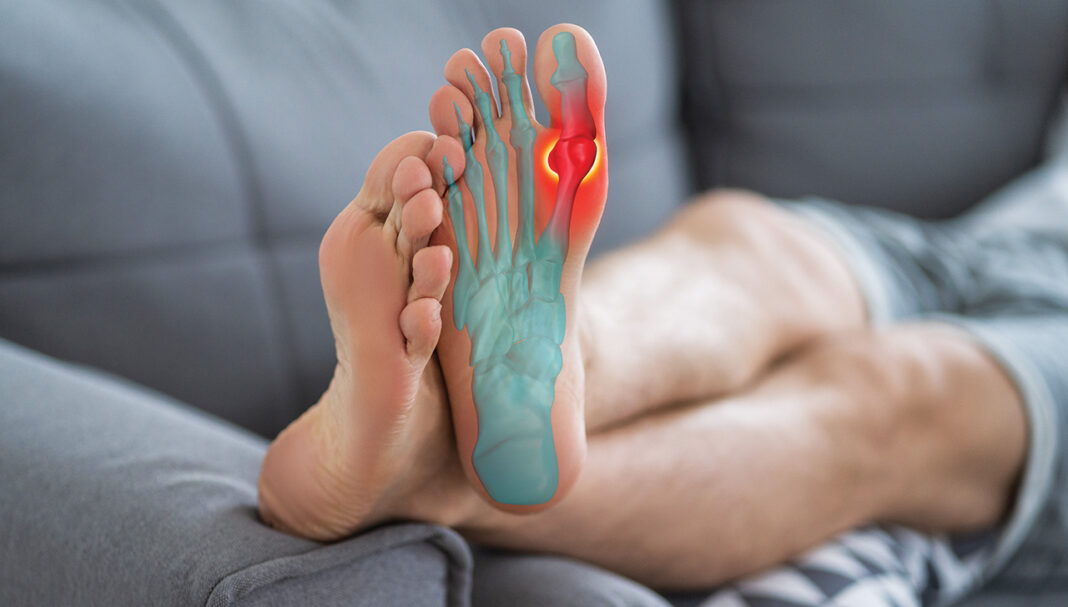
Darrel, a 67-year-old male, presents to your pharmacy with his regular prescription for allopurinol 300 mg/day, which he has taken for more than 2 years.
Darrel has a history of gout but no tophi and is frustrated that he continues to experience gout flares despite taking the allopurinol regularly. To treat his frequent gout flares, he usually takes ibuprofen. You look at the Therapeutic Guidelines and note that the dose of allopurinol should be titrated until target urate levels are achieved. You ask Darrel, and he does not recall having his urate levels measured, at least not recently.
Learning objectivesAfter reading this article, pharmacists should be able to:
Competency standards (2016) addressed: 1.1, 1.4, 1.5, 2.2, 3.1, 3.5. 3.6 |
THIS IS A CPD ARTICLE. YOU NEED TO BE A PSA MEMBER AND LOGGED IN TO READ MORE.